Located at Group 11 and Period 4 of the Periodic Table is one of the metal you will usually find in wires. This is Copper.
 |
| Christopher Columbus |
Copper is also used by Native Americans ( Early Americans, right before Christopher Columbus's discovery of American Continent ) in the New World ( First Named so when American Continent was first discovered by Christopher Columbus ) in 2000 BCE!
Because of copper's oldest discovery, copper was known to be the first alloy discovered by humans, discovered by 2000 BCE! Humans made copper alloy using tin and copper. These metals can be melted together easily and form an alloy. This alloy is called Bronze, also known as the Bronze Age.
The isolator is also unknown, since copper is so old! But it is said that the Mesopotamians, that stays at Mesopotamia, now known as the middle east. The region is now known to be Iraq, Kuwait, Turkey and Syria.
Mesopotamian history marked as one of the most important inventions in the world. Some of their inventions are the concept of time, sailboats, maps, writing, wheel and the most important of all : MATHS.
 |
| Mesopotamians Writing |
Mesopatamians learned how to use copper in weapons. Today, Copper is isolated in a more modern way - electrolysis! Electricity is passed through solutions containing copper compounds, such as copper sulfate. The anode, also known as the positive electrode is made from an impure copper and the cathode, also known as negative electrode is made from pure copper.
The insoluble impurities was then fall to the bottom. 99.99% purity of Copper was then made!
 |
| Chalcopyrite |
Copper can be isolated from its ores, such as chalcopyrite, chalcocite, covellite, bornite and more.
The most famous of copper usage is the statue of liberty, where the Statue of Liberty was made by France and given to United States as a gift in October 28, 1886.
 |
| Change of colors overtime |
United States penny used to be 95% of copper. In 1943, during World War 2, the penny became a huge mistake as the penny have zinc-plated steel. The penny got stuck in vending machine as steel is generally magnetic and zinc corrodes easily, causing the public to confuse it with a dime.
At 1980, copper became more valuable than the one cent, which is the penny was worth. So, the US mint decided to switch the penny's core to a much cheaper zinc core with copper coating.
Some interesting fact about copper is the blood. Human's blood contain oxygen, caused by oxyhaemogoblin ( a compound of haemogoblin combined with oxygen ). And the blood is red because it contains iron. Blood turns to purplish-red as it returns to the lungs because of the loss of oxygen.
Everything's not the same. Some animals do not have haemogoblins to carry oxygen around the blood. Such as the animal class Crustaceans, which have crabs, lobsters. shrimps and more. Crustaceans carry oxygen around the body using a compound named haemocyanin. Haemocyanin is just like haemogoblins but haemocyanin carries copper instead of iron. Most of the copper compounds are blue, and haemocyanin is one of them. And because of this, crustaceans' blood are blue. Yep, just like the nobility, blue-blooded.
 |
| Blue - Blooded Horse Shoe Crab |
However, I would suggest you to cut of the idea of cutting crustaceans apart for the look of blue blood in their bodies.
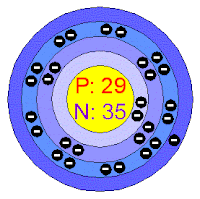
Atomic Number : 29
Name : Copper
Latin Name : Cuprum
Electrons per shell : [ 2, 8, 18, 1 ]
Discoverer : Unknown ( First used in 9000BCE! )
Isolator : Unknown ( No one knows who isolated Copper! )
Element's : Atomic Mass : 63.546 u
: Density : 8.96g/cm3
: Type : Transition Metal
Chemical Properties :
 |
| Copper - 64 |
- Corrosion Resistance
- When corroded, it forms a greenish tint
- Reacts with Oxygen
- Doesn't react with water
- When reacted with oxygen, it forms hydrated copper carbonate, also called as patina
- Dissolves in Acid, especially sulfuric acid
- Around 36 isotopes : 3 of them are :
Copper - 63 : Protons : 29
: Neutrons : 34
: Electrons : 29
( Half life : Stable )
Copper - 64 : Protons : 29
: Neutrons : 35
: Electrons : 29
( Half Life : 12.7 hours )
Copper - 65 : Protons : 29
: Neutrons : 36
: Electrons : 29
( Half Life : Stable )
- Soft
- Ductile
- Malleable
- Good conductor of electricity
- Good conductor of heat
- Reddish brown color
- Melting Point : 1083 degrees Celsius ( 1982 Fahrenheit )
- Boiling Point : 2595 degrees Celsius ( 4703 Fahrenheit )
How Copper got its name?
 |
| Cyprus Flag |
Uses
Copper's most important uses are for wiring. Almost every electrical device's wire are made from copper. You can take apart your phones or computers and find copper wiring around the circuit boards ( though we won't encourage you to take apart your electric devices ). The main reason copper is used is because copper is quite cheap and also a good conductor of electricity. And therefore, copper is used.
 |
| Copper Wires |
Some other compounds of copper is also used to make fungicides, such as Basic Copper Acetate, a compound to make insecticide and fungicide. Copper Methane Arsenate, used to make Algicide to kill algae.
THIS IS THE END OF COPPER
To go to homepage, click this link :
To go to Chemistry Element Page, click this link :
Thanks for visiting.
If there is any improvements needed, feel free to comment at the comment section below.
Thank you.
If there is any improvements needed, feel free to comment at the comment section below.
Thank you.





